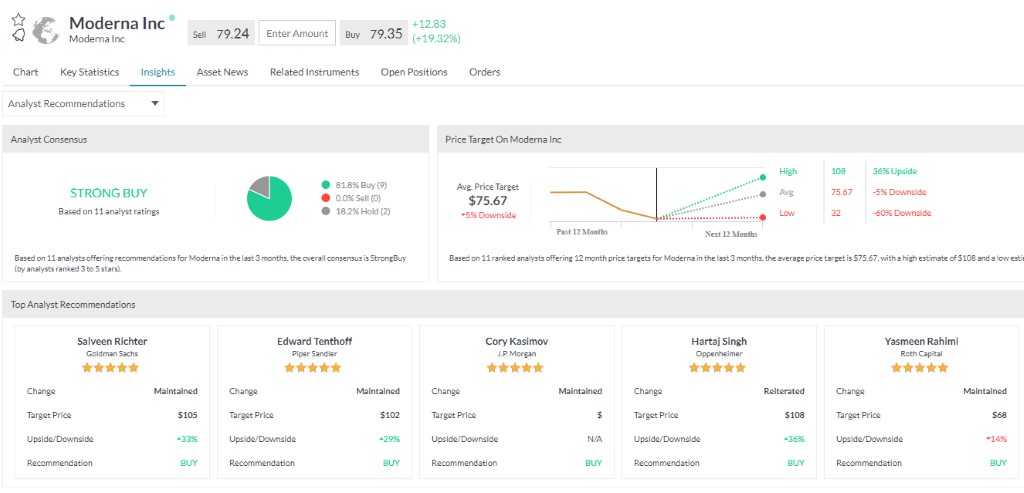
Webbplatsen share-dealing.markets.com/sv/ drivs av Safecap Investments Limited ("Safecap"), som regleras av CySEC under licens nr. 092/08. Safecap har sitt säte på 148 Strovolos Avenue, 2048, Strovolos, P.O.Box 28132, Nicosia, Cypern. Safecap Investments Limited är registrerat i Republiken Cypern under organisationsnummer ΗΕ186196.
Varning beträffande investeringar med hög risk: CFD:er är komplexa instrument och innebär en hög risk att snabbt förlora pengar på grund av hävstångseffekten. 77,3% av kontona för privata investerare förlorar pengar när de handlar med CFD:er hos denna leverantör. Du bör överväga om du förstår hur CFD:er fungerar och om du har råd att ta den höga risken att förlora dina pengar. Läs det fullständiga Uttalandet om riskinformation, som ger dig en mer detaljerad beskrivning av de involverade riskerna.
Beroende på landet för ditt medborgarskap eller permanenta bosättningsplats kan vi vara skyldiga, enligt gällande lokala lagar, regler och förordningar att erbjuda dig vissa ytterligare skyddsmekanismer (till exempel en garanterad stopp-förlustmekanism) eller införa ytterligare begränsningar på din handel. Vänligen läs noggrant vårt Serviceavtal för investeringar för mer information om sådana skydd eller begränsningar som kan tillämpas på dig.
Begränsade jurisdiktioner: Vi upprättar inte konton till invånare i vissa jurisdiktioner, bland annat Japan, Kanada, Belgien och USA. För mer information, se vårt Serviceavtal för investeringar.
Safecap Investments Limited ägs av Finalto (IOM) Limited.
För klagomål som rör integritet och dataskydd kan du kontakta oss på privacy@markets.com. Läs vår INTEGRITETSPOLICY för mer information om hanteringen av personuppgifter.
Markets.com är verksamt genom följande dotterbolag:
Markets South Africa (Pty) Ltd är reglerat av Financial Sector Conduct Authority ("FSCA") under licens nr 46860 och med tillstånd att verka som en leverantör av OTC-derivat i enlighet med Financial Markets Act nr 19 från 2012.
Finalto International Limited är registrerat i Saint Vincent och Grenadinerna ("SVG") enligt de reviderade lagarna i Saint Vincent och Grenadinerna 2009, med registreringsnummer 27030 BC 2023.